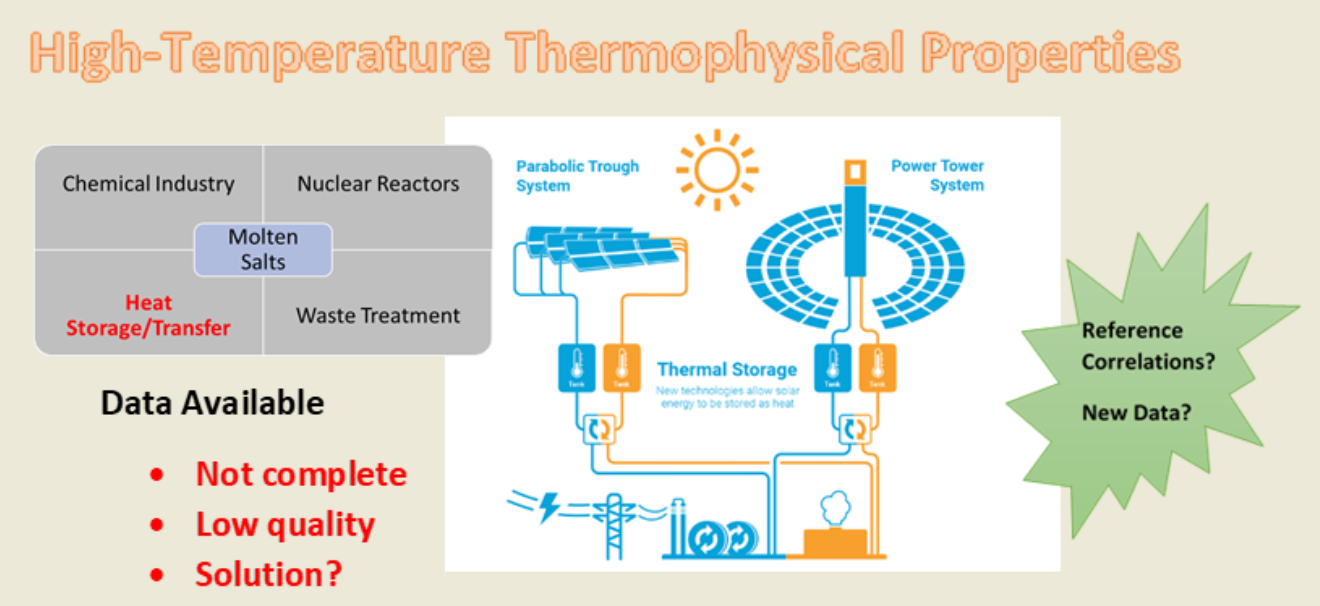
Molten salts are increasingly regarded as promising fluids for high-temperature heat transfer, thermal energy storage, and advanced reaction processes, including concentrated solar power (CSP), molten salt oxidation (MSO), and next-generation nuclear reactors. Among these materials, the ternary eutectic mixture Li₂CO₃–Na₂CO₃–K₂CO₃ (32.12–33.36–34.52 wt%) has emerged as a leading candidate due to its wide operating temperature range and favourable thermodynamic characteristics. Despite its relevance, substantial inconsistencies and gaps remain in the available thermophysical property data, posing challenges for reliable design, modelling, and industrial deployment.
This work revisits the Li₂CO₃–Na₂CO₃–K₂CO₃ eutectic through a critical assessment of the literature from its reported melting point at 397 °C (670 K) up to approximately 1200 K. Using a methodology inspired by IUPAC-supported strategies previously applied to common liquids such as water and hydrocarbons, we examine the quantity, quality, and coherence of existing measurements. Reference correlations are proposed only where the data are sufficiently robust to justify them.
The analysis highlights a pressing need for more accurate and comprehensive measurements—particularly for heat capacity, thermal conductivity, and viscosity—to enable the development of reliable standard reference correlations. Addressing these data deficiencies is essential for advancing the safe and efficient use of molten carbonates in high-temperature energy technologies.