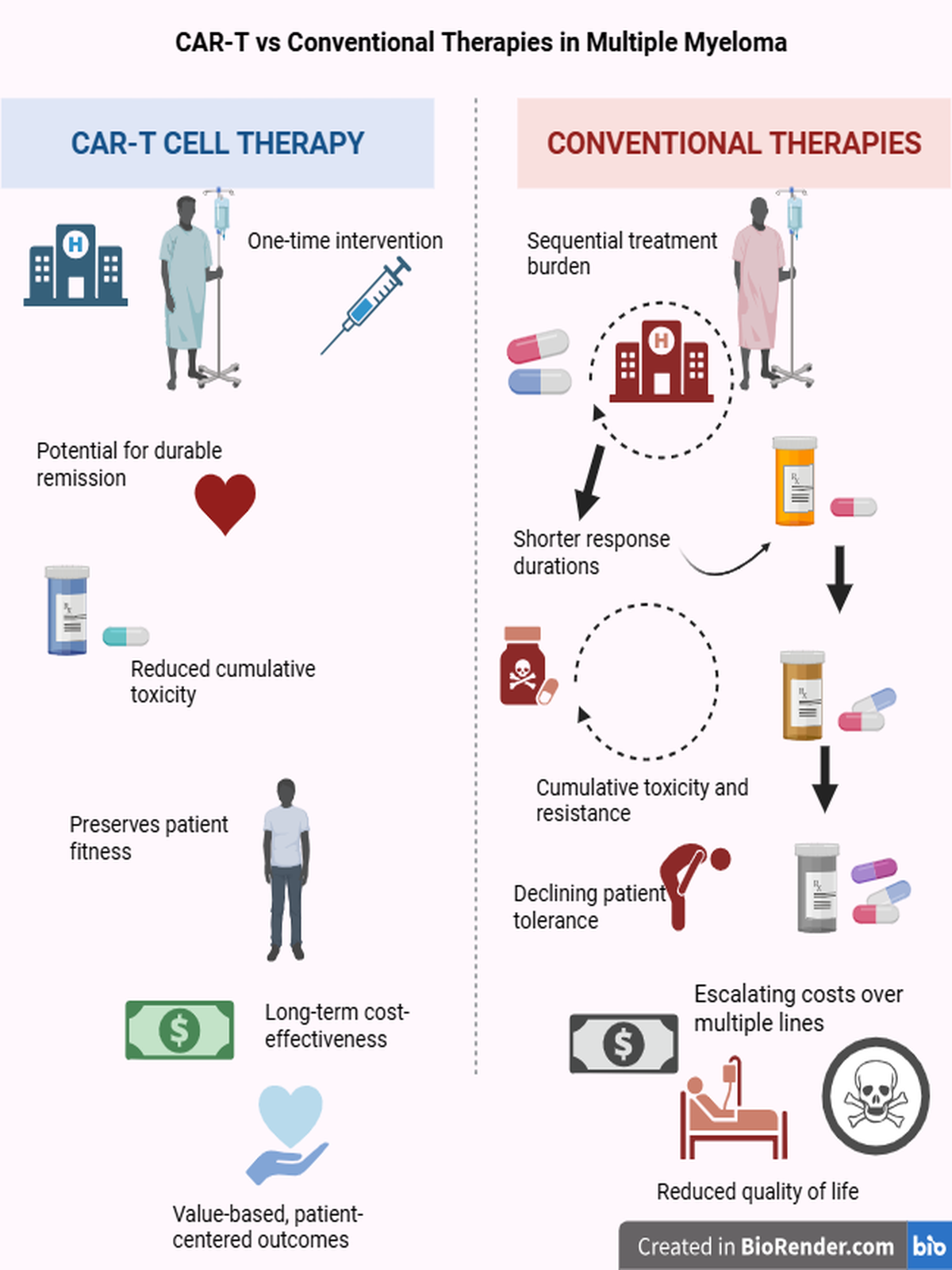
Background: Over the past century, numerous pharmacological regimens have been developed for multiple myeloma (MM), yet relapse remains inevitable. Although these regimens prolong overall survival (OS), repetitive treatment cycles and cumulative toxicity progressively impair quality of life (QoL). This study aimed to compare conventional stepwise therapies with CAR-T cell therapy in terms of QoL, toxicity, cost, and ethical value.Methods: Kaplan-Meier survival curves were analyzed to estimate overall (OS) and progression-free survival (PFS). Based on these durations, treatment costs were calculated. A simple and transparent utility-based model was developed to enable clinicians, researchers, and health policy authorities to easily estimate quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICER).Results: Among heavily pretreated patients, CAR-T therapy approximately doubled OS and PFS compared with other late-line regimens and provided markedly better quality of life with lower overall treatment burden. While daratumumab-based combinations improved survival and patient well-being, they were associated with very high treatment costs (~USD 1 million per patient). Carfilzomib-based regimens remained essential for managing high-risk disease despite their expense. In contrast, VMP represented a practical and accessible option, especially for transplant-ineligible or resource-limited patients.Conclusion: CAR-T therapy provided significant improvements in survival and quality of life compared with conventional regimens among patients who had received three or more prior lines of therapy. Its earlier use appears promising. However, the limited availability of CAR-T across only a few countries raises ethical concerns regarding treatment accessibility. Contrary to common assumptions, CAR-T can be less expensive than many traditional therapies, though its single-payment structure poses barriers for patients with limited financial means. Further analyses are needed to refine toxicity management and optimize its broader clinical application in multiple myeloma.